Factory source Isopropanol Factory - Ethanol CAS 64-17-5 Factory Direct Supply – Chemwin Detail:
Product Name:Ethanol
Molecular format:C2H6O
CAS No:64-17-5
Product molecular structure:
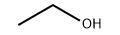
Chemical Properties:
Ethanol is highly soluble in water and organic solvents, but poorly soluble in fats and oils. Ethanol itself is a good solvent, which is used in cosmetics, paints and tinctures[2]. Density of ethanol at 68 °F (20 °C) is 789 g/l. Pure ethanol is neutral (pH ~7). Most alcoholic beverages are more or less acidic.
Ethanol/ethyl alcohol is highly flammable liquid, hygroscopic, and fully miscible in water. Ethanol is incompatible with a large number of chemicals such as strong oxidising agents, acids, alkali metals, ammonia, hydrazine, peroxides, sodium, acid anhydrides, calcium hypochlorite, chromyl chloride, nitrosyl perchlorate, bromine pentafluoride, perchloric acid, silver nitrate, mercuric nitrate, potassium tert-butoxide, magnesium perchlorate, acid chlorides, platinum, uranium hexafluoride, silver oxide, iodine heptafluoride, acetyl bromide, disulphuryl difluoride, acetyl chloride, permanganic acid, ruthenium (VIII) oxide, uranyl perchlorate, and potassium dioxide.
Application:
Medical
A solution of 70-85% of ethanol is commonly used as a disinfectant and it kills organisms by denaturing their proteins and dissolving their lipids. It is effective against most bacteria and fungi, and many viruses, but is ineffective against bacterial spores. This disinfectant property of ethanol is the reason that alcoholic beverages can be stored for a long time[9]. Ethanol also has many medical uses, and can be found in products such as medicines, medical wipes and as an antiseptic in most antibacterial hand sanitizer gels. Ethanal can also be used as antidote. It competitively blocks the formation of toxic metabolites in toxic alcohol ingestions by having a higher affinity for the enzyme Alcohol Dehydrogenase (ADH). Its chief application is in methanol and ethylene glycol ingestions. Ethanol can be administered by the oral, nasogastric or intravenous route to maintain a blood ethanol concentration of 100-150 mg/dl (22-33 mol/L).
Fuel
Ethanol is flammable and burns more cleanly than many other fuels. Ethanol has been used in cars since Henry Ford designed his 1908 Model T to operate on alcohol. In Brazil and the United States, the use of ethanol from sugar cane and grain as car fuel has been promoted by government programs[11]. The Brazilian ethanol program started as a way to reduce the reliance on oil imports, but it was soon realized that it had important environmental and social benefits[12]. The fully combusted products of ethanol are only carbon dioxide and water. For this reason, it is environmental friendly and has been used to fuel public buses in the US. However, pure ethanol attacks certain rubber and plastic materials and cannot be used in unmodified car engines.
The alcohol-based alternative fuel that is blended with gasoline to produce a fuel with a higher octane rating and fewer harmful emissions than unblended gasoline. A mixture containing gasoline with at least 10% ethanol is known as gasohol. Specifically, gasoline with 10% ethanol content is known as E10. Another common gasohol variant is E15, which contains 15% ethanol and 85% gasoline. E15 is only appropriate for use in Flex Fuel vehicles or a very small percentage of the newest vehicles[14]. In addition, E85 is a term used for a mixture of 15% gasoline and 85% ethanol. E85 keeps the fuel system clean because it burns cleaner than regular gas or diesel and doesn’t leave behind gummy deposits. Beginning with the model year 1999, a number of vehicles in the U.S. were manufactured so as to be able to run on E85 fuel without modification. These vehicles are often labeled dual fuel or flexible fuel vehicles, since they can automatically detect the type of fuel and change the engine’s behavior to compensate for the different ways that they burn in the engine cylinders.
The use of ethanol-diesel fuel blends is growing around the world, and are designed to provide renewable, cleaner burning fuel alternatives for off-road equipment, buses, semi-trucks and other vehicles that run on diesel fuel. With the addition of ethanol and other fuel additives to diesel, the characteristic black diesel smoke is eliminated and there are significant reductions in particulate matter, carbon monoxide, and nitrogen oxide emissions. It is also possible to use ethanol for cooking as a replacement for wood, charcoal, propane, or as a substitute for lighting fuels, such as kerosene.
Brazil and the United States lead the industrial production of ethanol fuel, accounting together for 89% of the world’s production in 2008. In comparison with the USA and Brazil, Europe ethanol for fuel production is still very modest. Brazil is the world’s second largest producer of ethanol fuel and the world’s largest exporter.
Beverage
Significant volumes of ethanol are produced for the beverage and industrial markets from agricultural feedstock. Ethanol produced for these industries differs from ethanol for fuel only in its strength, which can vary between 96% and 99.9% and in its purity, depending on the end use. Beverage and drinks industry may be the best-known end-user of ethanol. It is used to make many kinds of spirits, such vodka, gin and anisette. High standards and processes are required for ethanal used in the production of spirit drinks.
Others
The ethanol used as an intermediary product by the chemical, pharmaceutical or cosmetics industry is in many cases of the highest and purest possible quality. These are premium markets due to the additional steps in the alcohol production process that are necessary to achieve the required purity. Same high standards and purity requirements apply in food industry, such as flavors and aromas extraction and concentrations, as well as paints and thermometers. Ethanol can be used in de-icer or anti-freeze to clear the car windscreen. It also is contained in perfumes, deodorants, and other cosmetics
Product detail pictures:

Related Product Guide:
We have our own sales team, design team, technical team, QC team and package team. We have strict quality control procedures for each process. Also, all of our workers are experienced in printing field for Factory source Isopropanol Factory - Ethanol CAS 64-17-5 Factory Direct Supply – Chemwin , The product will supply to all over the world, such as: Southampton, Cologne, Chicago, Our stock have valued 8 million dollar , you can find the competitive parts within short delivery time. Our company is not only your partner in business, but also our company is your assistant in the coming corporation.
The supplier cooperation attitude is very good, encountered various problems, always willing to cooperate with us, to us as the real God.
Products categories
-

Phone
-

E-mail
-

Whatsapp
-

Top










