Product Name:Aniline
Molecular format:C6H7N
CAS No:62-53-3
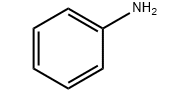
Product molecular structure:
Chemical Properties:
Chemical properties have alkaline, can be combined with hydrochloric acid to form hydrochloride, and with sulfuric acid to form sulfate. Can play the role of halogenation, acetylation, diazotization, etc. Flammable when exposed to open flame and high heat, and the flame of combustion will produce smoke. Strong reaction with acids, halogens, alcohols and amines will cause combustion. The N in the conjugated structure aniline is nearly sp² hybridized (actually it is still sp³ hybridized), the orbitals occupied by the lone pair of electrons can be conjugated with the benzene ring, the electron cloud can be dispersed on the benzene ring, so that the density of the electron cloud around the nitrogen is reduced.
Application:
Aniline is predominantly used as a chemical intermediate for dyes, drugs, explosives, plastics, and photographic and rubber chemicals. Many chemicals can be made from Aniline, including:
Isocyanaates for the urethane industry
Antioxidants, activators, accelerators, and other chemicals for the rubber industry
Indigo, acetoacetanilide, and other dyes and pigments for a variety of applications
Diphenylamine for the rubber, petroleum, plastics, agricultural, explosives, and chemical industries
Various fungacides and herbicides for the agricultural industry
Pharmaceutical, organic chemical, and other products
Products categories
-

Phone
-

E-mail
-

Whatsapp
-

Top













