China Cas 108-78-1 Polyethylene Terephthalate (PET) CAS 25038-59-9 High Quality And Low Price – Chemwin Detail:
Product Name:Polyethylene Terephthalate
Molecular format:C10H6O2
CAS No:25038-59-9
Product molecular structure:
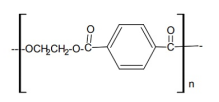
Chemical Properties:
Polyethylene terephthalate (PET) is a semi crystalline polymer possessing excellent chemical resistance, melt mobility and spinnability. The polymer is composed of repeating units as shown in the Figure 1. Each unit having a physical length of about 1.09 nm and a molecular weight of ~200. When it is produced from the reaction of terephthalic acid and ethylene glycol, it is capped on the left by H- and the right by –OH. Polymerization is thus accompanied by the production of water which is removed under elevated temperature and vacuum. Accordingly the presence of water in the molten state will rapidly depolymerize the structure so that thorough drying of the polymer prior to melt spinning of fibers is necessary.
The aromatic ring coupled with short aliphatic chain makes the polymer a stiff molecule as compared to other aliphatic polymers such as polyolefin or polyamide. The lack of segmental mobility in the polymer chains results in relatively high thermal stability. A textile grade polymer will have an average number of 100 repeat units per molecule so that the extended length of the typical polymer chain is about 100nm with a molecular weight about 20,000. Higher levels of polymerization produces higher strength fibers but the melt viscosity and stability of the melt to even tiny amounts of moisture causes hydrolytic degradation. The measurement of average degree of polymerization is done either by molten viscosity (by measuring the pressure drop through a calibrated orifice) or the viscosity of the diluted polymer in an appropriate solvent. The latter is measure of polymer chain length known as Intrinsic viscosity or IV and the value for a typical fiber grade polymer is 0.6 dl/g in 60/40 w/w mixture of phenol and tetrachloroethane solvent. The IV in the latter solvent is related to Mv (Viscosity average molecular weight) of the polymer by the Mark Howink equation (Equation 1).
Application:
Polyethylene terephthalate, commonly referred to by PET, is a member of the polyester family of polymers.The combination of properties like hardness and rigidity,dimensional stability, chemical resistance andlightweight, makes PET a flexible material widely used in several applications such as fibers, sheets, films,and beverage containers. Used in electrical parts including relay bases and lamp sockets, pump housings, gears, sprockets, chair arms, casters and furniture components.
Like other polyesters, PET is produced industrially by direct esterification of dicarboxylic acids with diols.Typical production processes are based on polymerization of ethylene glycol (MEG) with either purifiedterephthalic acid (PTA) or dimethyl terephthalate (DMT) in the presence of a metal catalyst.
Product detail pictures:

Related Product Guide:
High quality Initial,and Buyer Supreme is our guideline to offer the ideal assistance to our shoppers.At present, we are striving our best to become amongst the ideal exporters inside our industry to satisfy shoppers more want for China Cas 108-78-1 Polyethylene Terephthalate (PET) CAS 25038-59-9 High Quality And Low Price – Chemwin , The product will supply to all over the world, such as: Sao Paulo, Brunei, Uruguay, The design, processing, purchasing, inspection, storage, assembling process are all in scientific and effective documentary process , increasing usage level and reliability of our brand deeply, which makes us become superior supplier of the four major product categories shell castings domestically and obtained the customer's trust well.
This company has a lot of ready-made options to choose and also could custom new program according to our demand, which is very nice to meet our needs.
Products categories
-

Phone
-

E-mail
-

Whatsapp
-

Top










